The Food and Drug Administration says there’s an unmet need for help for patients suffering from severe sickle cell anemia.
A new drug that uses gene editing could solve that.
Sickle cell anemia patients have described the pain, exhaustion, and stress they face with their disease.
"Part of it is the excruciating pain that you have. You can't predict it ... I mean, you feel like just knives, knives, start stabbing you like everywhere, whenever you're hurting," said Travis Robinson.
"I describe it as glass breaking in my body ... I had a crisis actually every month throughout the year last year, my sophomore year of high school, and I had some teachers that were also not very understanding," said Ayana Johnson.
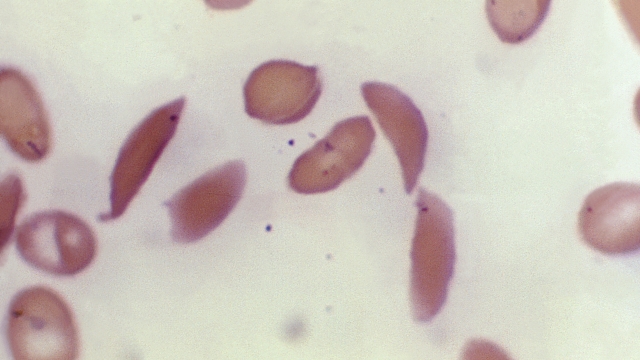
Normal red blood cells look fully round and carry oxygen to the body. Because of a gene mutation, sickle cell anemia causes those red blood cells to break down early — taking a sickle or c-shape that carries less oxygen. It can lead to blocked blood flow, pain, infection, and organ failure.
The CDC says 100,000 Americans are living with sickle cell anemia, 1 in every 365 Black or African American births.
Because of the cell destruction, patients only live to an average to 52 years old. Right now, bone marrow or stem cell transplants are the only FDA-approved cures for sickle cell anemia.
This week FDA advisers debated an IV infusion called Exa-cel from drugmakers CRISPR Therapeutics and Vertex. It would be the first treatment that uses CRISPR technology that allows scientists to change DNA.
The group recommended it, but shared some concerns about the research.
One complaint was that the research's patient groups were small. And there were additional concerns about the gene-editing targeting incorrectly, which can cause severe effects.
"These cell products have lots of cells in them, hundreds of millions of cells. And any one cell that goes awry, you know, could cause leukemia. Now, has that ever been shown that an off-target effect of gene editing causes leukemia? No," said Dr. Daniel Bauer, principal investigator and staff physician at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center at Boston Children’s Hospital.
Researchers plan to follow patients for the first 15 years after treatment.
The FDA is expected to decide on approval by the end of the year, and typically follow the guidance of its advisers.
SEE MORE: WXYZ: Red Cross Urges African American Donors To Give Blood
Trending stories at Scrippsnews.com